HPV+ Recurrent or Metastatic Head and Neck Cancer
Human papilloma virus (HPV)-positive cancers account for more than 20,000 deaths each year in the U.S. and Europe. The majority of these cancers are driven by HPV 16 which carries the E7 antigen targeted by CUE-101. Despite treatment with current standards of care the majority of patients with metastatic disease will experience recurrence, significantly affecting quality of life and often leading to untimely death. Patients with HPV-related head and neck, cervical and other HPV-driven cancers represent an important unmet clinical need and underscore the opportunity for promising new therapeutics.
Phase 1 Dose-Escalation and Expansion Trial of CUE-101 as Monotherapy in HPV+ Recurrent or Metastatic Head and Neck Cancer
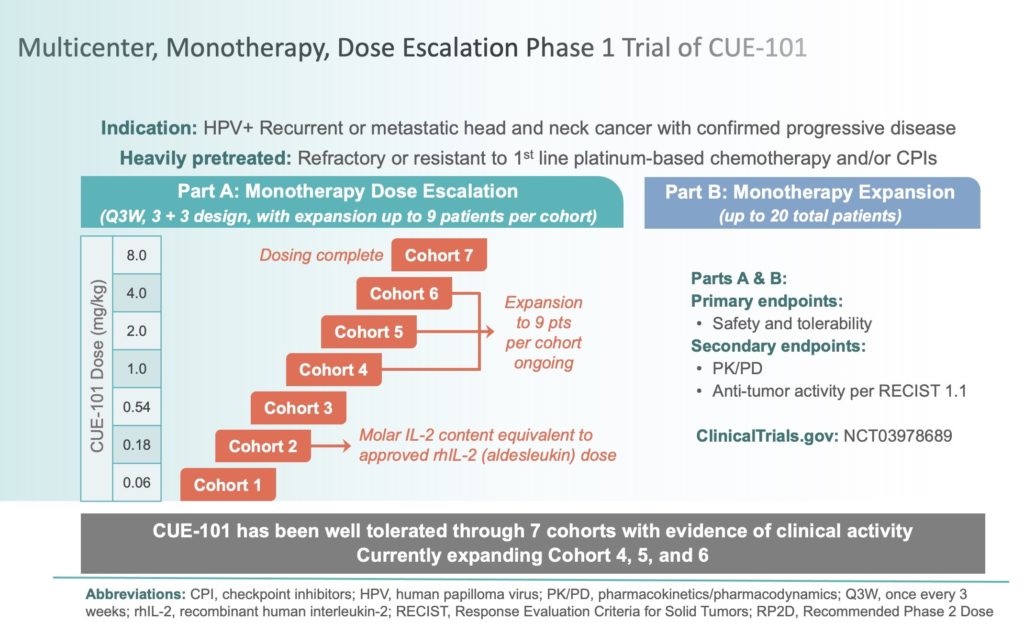
In September 2019, we initiated a first-in-human, multi-center, open-label, Phase 1 dose-escalation and dose-expansion study of CUE-101, our lead drug candidate, for eligible patients with recurrent or metastatic human papilloma virus-positive head and neck squamous cell carcinoma (HPV+ R/M HNSCC). This monotherapy trial is focused on second line or later (2L+) R/M HNSCC patients, which have failed prior therapies including the application of immune checkpoint inhibitors. CUE-101 is a novel fusion protein that incorporates HLA-A*0201 bound to an epitope from the HPV 16 E7 protein (E711-20) and is designed to activate and expand tumor-specific T cells that target Human Papilloma Virus 16 (HPV16)-driven malignancies. Cue Biopharma has engaged a network of nationally recognized clinical investigators and 14 Phase 1 sites are actively recruiting patients.
Eligible patients with HPV+ R/M HNSCC will have progressive disease after at least one prior systemic therapy, including a platinum-based chemotherapy, and were offered and/or been administered checkpoint immunotherapy. In addition, patients must have (1) histologically and/or cytologically confirmed HPV16+ tumors, and (2) an HLA-A*0201 genotype as determined by genomic testing.
The primary objectives of this Phase 1 trial are to assess the safety, tolerability, and preliminary anti-tumor activity following CUE-101 administered intravenously every three weeks in patients suffering with HPV+ R/M HNSCC. The frequency of all adverse events, including any that are believed to be treatment related, will be assessed, with the objectives of identifying the maximum tolerated dose (MTD) and recommended Phase 2 dose (RP2D) and monitoring for the possible generation of anti-drug antibodies (ADAs) and cytokine-release syndrome.
Pharmacokinetics (PK) of CUE-101 administration will also be evaluated, including dose-dependency of drug exposure and elimination half-life with single and repeated infusions. To determine if CUE-101 is having its predicted effect on T cells, selective expansion and activity E7 responsive T cells will be monitored
By mid-January 2021, this Phase 1 trial had progressed through seven cohorts with CUE-101 doses ranging from 0.06 mg/kg in Cohort 1 to 8.0 mg/kg in Cohort 7 administered by IV infusion once every three weeks. To date, CUE-101 has been well-tolerated and it has demonstrated dose-proportional pharmacokinetics.
For additional information on the trial protocol please see (NCT03978689).
Phase 1 Dose-Escalation and Expansion Trial of CUE-101 in Combination with KEYTRUDA® (pembrolizumab) as First-Line Therapy in HPV+ Recurrent or Metastatic Head and Neck Cancer
In early February 2021, we dosed the first patient with newly diagnosed recurrent or metastatic HPV+ HNSCC in a multi-center, open-label, Phase 1 dose-escalation and dose-expansion study of CUE-101 in combination with Keytruda® (pembrolizumab) at its recommended dose of 200 mg. The first dose cohort will be administered 1.0 mg/kg CUE-101, the same dose as Cohort 4 in the monotherapy portion of this trial. Pembrolizumab and CUE-101 will be dosed sequentially every 3 weeks. This combination trial is an amendment to the monotherapy trial described above (NCT03978689) and is referred to as Parts C and D. It is being conducted in collaboration with Merck & Co., Inc. and is also referred to as KEYNOTE-A78.
The goal of the Part C and D combination trial is to characterize the safety, tolerability, and biological effects of CUE 101 in combination with pembrolizumab as first line therapy in patients with unresectable, recurrent or metastatic HNSCC whose tumors express PD-L1 (Combined Positive Score [CPS] ≥1 as determined by an FDA-approved test). Eligible patients with recurrent or metastatic HPBV+ HNSCC will have been considered incurable by local therapies (i.e., surgery and/or radiation). This is the same patient population treated in KEYNOTE-A78.
KEYNOTE-A78 is being conducted in the U.S. at the same 14 clinical sites as the monotherapy portion of this trial. It is expected to recruit up to 35 patients.
The primary endpoints include the number of subjects with dose limiting toxicities (DLTs) and the pharmacokinetics of CUE-101 in combination with pembrolizumab. The secondary endpoint is the overall response rate (ORR) per RECIST 1.1. Complete and partial responses and stable disease parameters will be followed.
Phase 1 Neoadjuvant Trial of CUE-101 in Newly Diagnosed Patients with HPV+ Head and Neck Cancer
In the second half of 2021, Cue Biopharma expects to commence a Phase 1 neoadjuvant trial of CUE-101 in newly diagnosed patients with HPV+ head and neck cancer that are scheduled for definitive therapy with surgery or radiation. The objective of this trial is to obtain evidence at the cellular level that CUE-101 activated E7-specific CD8+ T cells are capable of infiltrating tumors to identify and eliminate tumor cells. The proportion of viable and necrotic tumor cells before and after CUE-101 treatment will be measured.

